
RESOURCES
What’s Happening in AAV Gene Therapy Now
-
 resourcesWhen every second counts, shaving days from a gene therapy manufacturing timeline is a huge win. What if you could eliminate months? Watch this Cell & Gene webinar where Viralgen’s Ane Quesada, PhD,...
resourcesWhen every second counts, shaving days from a gene therapy manufacturing timeline is a huge win. What if you could eliminate months? Watch this Cell & Gene webinar where Viralgen’s Ane Quesada, PhD,... -
 resourcesThe US Food and Drug Administration’s Office of Therapeutic Products (OTP) convened a public listening session on September 18, 2025, asking the cell and gene therapy (CGT) community how prior...
resourcesThe US Food and Drug Administration’s Office of Therapeutic Products (OTP) convened a public listening session on September 18, 2025, asking the cell and gene therapy (CGT) community how prior... -
 resourcesIn October, Andy Holt, Chief Commercial Officer at Viralgen, provided attendees of Meeting on the Mesa 2025 with an update on three of the company’s latest initiatives to help innovators advance...
resourcesIn October, Andy Holt, Chief Commercial Officer at Viralgen, provided attendees of Meeting on the Mesa 2025 with an update on three of the company’s latest initiatives to help innovators advance... -
 resources“May you live in interesting times.” Viralgen Chief Commercial Officer Andy Holt invokes that apocryphal “half-blessing, half-curse” as he opens his recent appearance on Outsourced Pharma Capacity...
resources“May you live in interesting times.” Viralgen Chief Commercial Officer Andy Holt invokes that apocryphal “half-blessing, half-curse” as he opens his recent appearance on Outsourced Pharma Capacity... -
resourcesIn July, a panel of expert speakers—Christine Lebec (Sensorion), Leigh Shaw (SpliceBio), Nathalie Clement (Siren Biotechnology), and María Orío (Viralgen)—came together to explore the evolving...
-
 resourcesViralgen is streamlining gene therapy manufacturing with a platform approach that accelerates timelines and reduces complexity. By leveraging process knowledge across multiple AAV serotypes, we...
resourcesViralgen is streamlining gene therapy manufacturing with a platform approach that accelerates timelines and reduces complexity. By leveraging process knowledge across multiple AAV serotypes, we... -
 resourcesAt Viralgen, we’re tackling one of the biggest challenges in gene therapy manufacturing: scaling AAV production from research to GMP. By analyzing historical data and applying percentile-based...
resourcesAt Viralgen, we’re tackling one of the biggest challenges in gene therapy manufacturing: scaling AAV production from research to GMP. By analyzing historical data and applying percentile-based... -
resourcesIn the race to deliver gene therapies to patients faster, every step of the manufacturing process counts. Viralgen is applying multivariate data analysis (MVDA) to boost upstream AAV production,...
-
resourcesAt the forefront of gene therapy innovation, Viralgen is redefining how advanced therapy medicinal products (ATMPs) are manufactured. “Streamlining Advanced Therapy Medicinal Product Manufacturing: A...
-
resourcesAs gene therapies continue to revolutionize medicine, manufacturers are under pressure to produce adeno-associated virus (AAV) vectors more efficiently and at higher quality. Viralgen is tackling...
-
 resourcesLive30 webinars are thirty-minute presentations designed to update you on the latest innovations, applications, and data in a fast yet interactive format.
resourcesLive30 webinars are thirty-minute presentations designed to update you on the latest innovations, applications, and data in a fast yet interactive format. -
.png) resourcesDisplacement Chromatography for Enrichment of rAAV Genome-Containing Capsids using Weak Organic AcidRecombinant adeno-associated virus (rAAV) vectors have become essential in gene therapy for delivering therapeutic genes effectively. However, the challenge of separating empty capsids from...
resourcesDisplacement Chromatography for Enrichment of rAAV Genome-Containing Capsids using Weak Organic AcidRecombinant adeno-associated virus (rAAV) vectors have become essential in gene therapy for delivering therapeutic genes effectively. However, the challenge of separating empty capsids from... -
 resourcesRevolutionizing recombinant adeno-associated virus (rAAV) characterization, Viralgen’s poster presents how high-throughput sequencing (HTS) enables detailed insights into rAAV product integrity,...
resourcesRevolutionizing recombinant adeno-associated virus (rAAV) characterization, Viralgen’s poster presents how high-throughput sequencing (HTS) enables detailed insights into rAAV product integrity,... -
 resourcesNavigating the complexities of Chemistry, Manufacturing, and Controls (CMC) regulations is critical for advancing gene therapies. Viralgen’s latest poster highlights their innovative approach to...
resourcesNavigating the complexities of Chemistry, Manufacturing, and Controls (CMC) regulations is critical for advancing gene therapies. Viralgen’s latest poster highlights their innovative approach to... -
 resourcesHigh-throughput sequencing (HTS) is transforming recombinant adeno-associated virus (rAAV) characterization, and Viralgen's latest poster showcases cutting-edge methodologies for deeper insights into...
resourcesHigh-throughput sequencing (HTS) is transforming recombinant adeno-associated virus (rAAV) characterization, and Viralgen's latest poster showcases cutting-edge methodologies for deeper insights into... -
 resourcesIn the rapidly evolving field of gene therapy, optimizing the production of adeno-associated viral vectors (AAVs) is essential for success. Our latest white paper dives into the advantages of using...
resourcesIn the rapidly evolving field of gene therapy, optimizing the production of adeno-associated viral vectors (AAVs) is essential for success. Our latest white paper dives into the advantages of using... -
 resourcesInnovations in adeno-associated virus (AAV) manufacturing are shaping the future of gene therapy, and Viralgen's recent scientific poster highlights a groundbreaking approach. The study demonstrates...
resourcesInnovations in adeno-associated virus (AAV) manufacturing are shaping the future of gene therapy, and Viralgen's recent scientific poster highlights a groundbreaking approach. The study demonstrates... -
 resourcesIn the rapidly evolving field of gene therapy, process optimization is key to success. Viralgen's latest poster reveals how multivariate data analysis (MVDA) enhances upstream AAV vector...
resourcesIn the rapidly evolving field of gene therapy, process optimization is key to success. Viralgen's latest poster reveals how multivariate data analysis (MVDA) enhances upstream AAV vector... -
 resourcesViralgen relies on a proprietary manufacturing platform, advanced applications of data, and broad experience to accelerate production
resourcesViralgen relies on a proprietary manufacturing platform, advanced applications of data, and broad experience to accelerate production -
 resourcesDiscover how Viralgen's Regulatory Affairs team drives faster speed to clinic and improved product quality through proactive collaboration with customers and regulators. This whitepaper explores how...Read More about Enhancing CMC Regulatory Efficiency in Gene TherapySeptember 10, 2024
resourcesDiscover how Viralgen's Regulatory Affairs team drives faster speed to clinic and improved product quality through proactive collaboration with customers and regulators. This whitepaper explores how...Read More about Enhancing CMC Regulatory Efficiency in Gene TherapySeptember 10, 2024 -
.png) resourcesExplore how TAAV and Viralgen simplify rAAV manufacturing with neDNA™ and the Pro10™ platform. This whitepaper highlights how these innovative technologies accelerate gene therapy development, reduce...
resourcesExplore how TAAV and Viralgen simplify rAAV manufacturing with neDNA™ and the Pro10™ platform. This whitepaper highlights how these innovative technologies accelerate gene therapy development, reduce... -
 resourcesDiscover key strategies for overcoming variability and scalability challenges in AAV production for gene therapy. This poster by Viralgen highlights comparability studies, process changes, and...
resourcesDiscover key strategies for overcoming variability and scalability challenges in AAV production for gene therapy. This poster by Viralgen highlights comparability studies, process changes, and... -
resourcesAs gene therapy continues to evolve, overcoming the variability and scaling challenges in AAV manufacturing is crucial for the production of safe and effective treatments. Manufacturers face...
-
 resourcesThis study highlights the importance of monitoring viral capsid protein charge distribution in AAV gene therapy vectors to ensure product quality. Using imaged capillary isoelectric focusing (icIEF),...
resourcesThis study highlights the importance of monitoring viral capsid protein charge distribution in AAV gene therapy vectors to ensure product quality. Using imaged capillary isoelectric focusing (icIEF),... -
 resourcesNavigating regulatory challenges with Viralgen’s head of regulatory affairs María OríoRead More about Addressing Barriers in Gene TherapyDecember 18, 2023
resourcesNavigating regulatory challenges with Viralgen’s head of regulatory affairs María OríoRead More about Addressing Barriers in Gene TherapyDecember 18, 2023 -
resourcesThe rapid advancement of cell and gene therapies offers unprecedented potential to treat complex diseases, but navigating both scientific and financial challenges remains a significant hurdle. From...
-
-1.png) resourcesThis study compares the production of recombinant AAV vectors using traditional plasmid DNA and neDNA™, a linear DNA molecule free of bacterial sequences. Evaluating both methods in Viralgen’s Pro10™...
resourcesThis study compares the production of recombinant AAV vectors using traditional plasmid DNA and neDNA™, a linear DNA molecule free of bacterial sequences. Evaluating both methods in Viralgen’s Pro10™... -
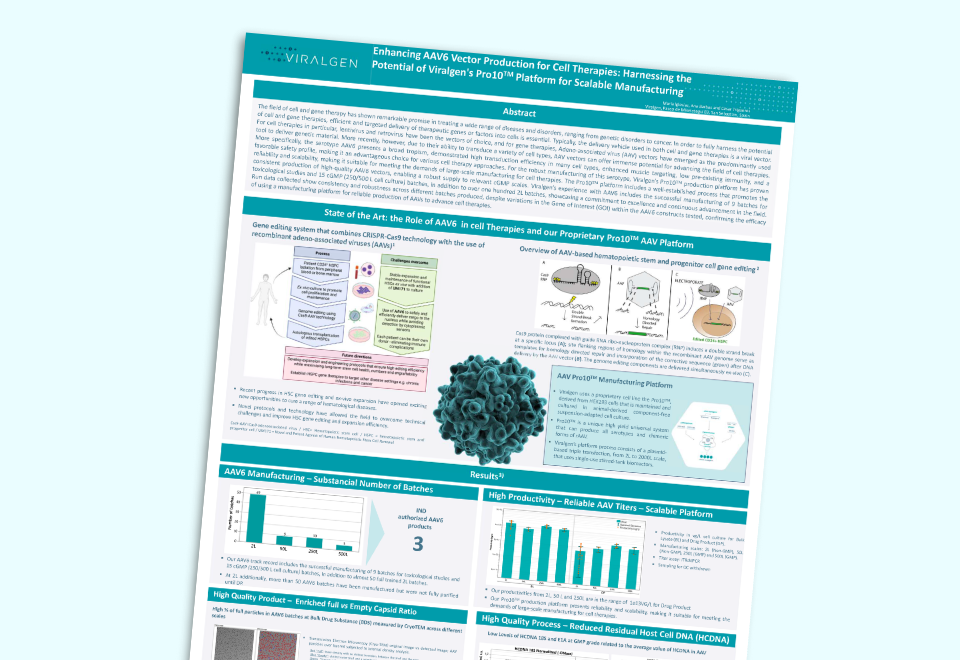 resourcesViralgen leverages its Pro10™ production platform to manufacture high-quality AAV6 vectors, enabling efficient, large-scale production for cell therapies. With broad tropism, high transduction...
resourcesViralgen leverages its Pro10™ production platform to manufacture high-quality AAV6 vectors, enabling efficient, large-scale production for cell therapies. With broad tropism, high transduction... -
.png) resourcesViralgen offers specialized production of Adeno-Associated Virus (AAV) gene therapy vectors, using a proprietary suspension and triple transfection platform. Our scalable 2000L manufacturing process...
resourcesViralgen offers specialized production of Adeno-Associated Virus (AAV) gene therapy vectors, using a proprietary suspension and triple transfection platform. Our scalable 2000L manufacturing process... -
 resourcesSuccessful technology transfer is essential for scaling gene therapy manufacturing processes from clinical to commercial stages. This poster highlights Viralgen’s proven methodology for ensuring a...
resourcesSuccessful technology transfer is essential for scaling gene therapy manufacturing processes from clinical to commercial stages. This poster highlights Viralgen’s proven methodology for ensuring a... -
 resourcesDespite recent advances in the gene therapy field, significant challenges lie ahead for the consistent manufacturing and release of these medicines. rAAV particles are built from the folding of three...
resourcesDespite recent advances in the gene therapy field, significant challenges lie ahead for the consistent manufacturing and release of these medicines. rAAV particles are built from the folding of three... -
 resourcesViralgen developed a dedicated platform and a designed-for-use cell line, which combine to improve efficiency and reduce costs
resourcesViralgen developed a dedicated platform and a designed-for-use cell line, which combine to improve efficiency and reduce costs -
 resourcesJoin this webinar to examine scale-up optimizations of AAV gene therapy in upstream processing. The featured speaker will explore both process characterization and experimental approaches to better...
resourcesJoin this webinar to examine scale-up optimizations of AAV gene therapy in upstream processing. The featured speaker will explore both process characterization and experimental approaches to better... -
 resourcesDiscover how the HEK293-derived Pro10™ cell line accelerates AAV vector production. This whitepaper explores a scalable, animal component-free platform that reduces process development time and...
resourcesDiscover how the HEK293-derived Pro10™ cell line accelerates AAV vector production. This whitepaper explores a scalable, animal component-free platform that reduces process development time and...
PREV
NEXT





