Aava™: Your name to know for premier AAV gene therapy manufacturing
Aava is the high-performance, real-world-ready AAV manufacturing platform that can get your high-potential gene therapies to market quickly and efficiently.


The gene therapy manufacturing platform up to every challenge
Aava exists because Viralgen is all in on AAV—it’s all we do, all day, every day. As a result, our manufacturing platform, and our Pro10™ cell line, have amassed an impressive 15-years-and-counting record of success for biotech and pharmaceutical partners large and small, from early construct optimization to small feasibility studies to full-on commercial campaigns.
We’ve produced more than 30 AAV serotypes, created upwards of 1,500 AAV batches, and been involved in over four dozen clinical trials.
All of which means you can count on Aava to deliver the perfect combination of speed, scale, predictability, and performance to maximize your opportunities to get life-changing therapies to patients.
Pro10™ Cell Line
Proprietary Pro1O™ is an animal origin-free, suspension-adapted cell line, derived from HEK293 cells. Pro1O™ is a unique, high-yield universal system that can produce all serotypes and chimeric forms of rAAV.
Upstream Process
Plasmid-based triple transfection, 50 to 2000L, single-use stirred-tank bioreactors
Downstream Process
Viralgen has the capability of processing 50 to 2000L batches with depth filtration, affinity and ion exchange chromatography and tangential flow filtration, as well as ultracentrifugation for full/empty separation.
Fill & Finish
Fully automated isolator
QC Release & CMC
>80% performed in-house
Put Aava to the test.
Want to see exactly what Aava can do for your AAV-based gene therapy projects?
Proven cell line technology optimized for high yields
Gene therapies will find their clearest, shortest paths to success when manufacturing processes can reliably deliver increased production and lower costs. That’s precisely why our Aava manufacturing platform relies on Pro10™, a proprietary cell line technology licensed exclusively from AskBio.
Introduced in 2010, Pro10™ has demonstrated best-in-class speed to scale (think six months to cGMP instead of 18 months), so timelines can be met without compromising quality. Derived from HEK293 cells and maintained and cultured in serum-free suspension media, Pro10™ is a unique high-yield universal technology that our team has used to produce more than 30 AAV serotypes.



What can Aava do for you? Exactly what you need.
Each generation of Aava is fine-tuned to meet specific strategic needs, whether that’s reducing risk on your way into the clinic or pushing efficiency up to drive costs down.
And every generation is developed through a process of continuous improvement, so no matter which path you choose, you can count on implementing the most advanced and innovative solution.
Speed + Stability
Pro10™ Cell Line
Helper Plasmid DNA
250-2000L cGMP Production
DNAse-Free Harvest
Downstream Ultracentrifugation

Scale + Efficiency
Pro10™ Cell Line
Helper Plasmid DNA
Upstream High Cell Density Perfusion
DNAse-Free Harvest
Downstream AEX Chromatography

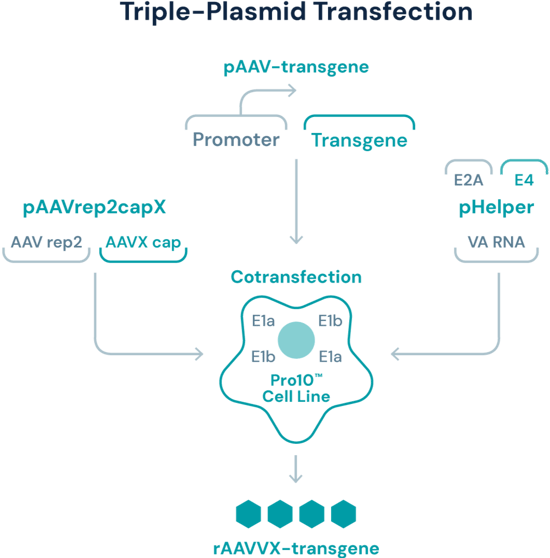
Triple transfection crafted to multiply upstream efficiencies
At Viralgen, we’ve optimized RepCap and helper plasmids for use as a part of our Aava platform. After expansion, Pro10™ cells are co-transfected with: (a) the transgene plasmid, (b) a plasmid carrying pAAVrep2capX; and (c) a helper plasmid carrying the adenoviral genes needed for AAV production. The resulting triple transfection delivers valuable functional and production advantages, while simultaneously enhancing safety by preventing unwanted viral genomes from replicating.
Aava: Everything you’re looking for in an AAV platform.
Better Predictability
Aava has been used to produce 1,500+ batches — meaning all of that data can be used as a “digital bioreactor” to signal early predictability of your construct
Superior
Speed
Aava offers fast scalability from research to clinical to commercial, all while reducing your process development and validation timelines
Deeper
Data
Aava delivers value where it counts: Access to our CMC package and data management simplifies and de-risks the registration process

Aava’s story by the numbers.



Ready to Start?
Process matters. We’re confident you’ll like ours.
Discover it firsthand by connecting with our team and describing what gene therapy manufacturing hurdles you want to clear first.





