Maximize your efficiency through a gene therapy manufacturing platform up to every challenge
Put tested, trusted AAV manufacturing to work – the kind used to produce 1,500 batches and counting

A platform designed and optimized for high yields
Gene therapies will find their clearest, shortest paths to success when manufacturing processes can reliably deliver increased production and lower costs. That’s precisely why our AAV manufacturing platform relies on Pro10™, a proprietary cell line technology licensed exclusively from AskBio.
Introduced in 2010, Pro10™ has demonstrated best-in-class speed to scale (think six months to cGMP instead of 18 months), so timelines can be met without compromising quality. Derived from HEK293 cells and maintained and cultured in serum-free suspension media, Pro10™ is a unique high-yield universal technology that our team has used to produce more than 30 AAV serotypes.
.png?width=644&height=873&name=Group%2062%20(3).png)
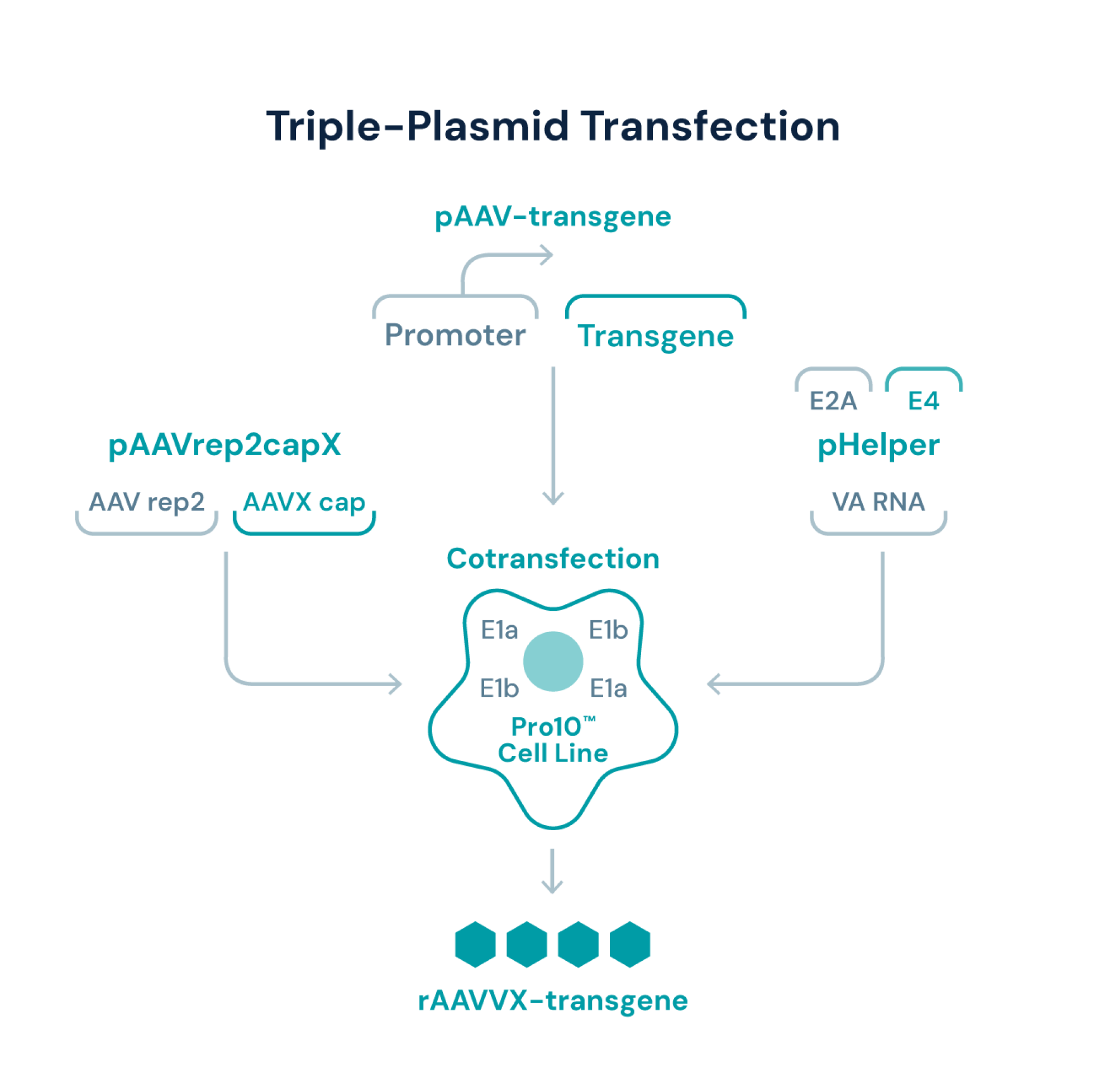
Triple transfection crafted to multiply upstream efficiencies
At Viralgen, we’ve optimized RepCap and helper plasmids for use as a part of our platform approach to upstream manufacturing. After expansion, Pro10™ cells are co-transfected with: (a) the transgene plasmid, (b) a plasmid carrying pAAVrep2capX; and (c) a helper plasmid carrying the adenoviral genes needed for AAV production. The resulting triple transfection delivers valuable functional and production advantages, while simultaneously enhancing safety by preventing unwanted viral genomes from replicating.

Manufacturing expertise that’s always ready, willing and able to advance your next step
Given the time and expense traditionally involved, it’s only natural for the gap between cell line development and regulatory approval to feel unbridgeable. Unless, that is, you have an experienced gene therapy manufacturing team like Viralgen at your side every step of the way.
In our San Sebastián facilities – the world’s largest devoted to AAV-based gene therapy manufacturing – more than 400 professionals combine their expertise in cell line development, upstream and downstream processes, fill and finish, and QC and release to help our clients advance their programs efficiently. Put simply, our platform approach works: it minimizes your time and cost of process development and validation, all while delivering you the confidence that can only come from collaborating with a proven, market-validated partner.
Stand steady on a stronger platform
Toxicology batches, clinical cGMP batches, engineering runs – we’ve aced gene therapy manufacturing at every volume. Along the way, we’ve applied that experience in a way that positions you to capitalize on every advantage our platform delivers today.
BETTER PREDICTABILITY
Our platform has been used to produce 1,500+ batches – meaning all of that data can be used as a “digital bioreactor” to signal early predictability of your construct
SUPERIOR SPEED
Our platform offers fast scalability from research to clinical to commercial, all while reducing your process development and validation timelines
DEEPER DATA
Our platform delivers value where it counts: Access to our CMC package and data management simplifies and de-risks the registration process

A manufacturing partner ready to grow with you
Let’s start a conversation about everything you need from your gene therapy manufacturing partner as you scale your program from discovery to clinic and beyond. We’ll tell you everything we can deliver, and then we can discover together if there’s a good match for what you have ahead.